Chinese Scientists Pioneer Sugar Synthesis from Carbon Dioxide
This breakthrough may provide a stable new supply route for this vital ingredient.
A team of researchers at the Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences has achieved a significant breakthrough in artificial sugar synthesis, marking a substantial step towards resolving the growing concerns about the safety and risks of sugar supply.
A Leap from Dependence on Sugary Biomass Resources
Traditionally, sugar extraction and production has relied heavily on plant-based biomass resources. This process, from carbon dioxide to biomass resources to sugar, is inhibited by the energy conversion efficiency of plant photosynthesis. Moreover, challenges such as land degradation and scarcity, ecosystem degradation, and extreme weather conditions and natural disasters caused by global warming have posed considerable risks to this production model.
To address these issues, the scientific community has been exploring the shift from this traditional sugar-making process to non-sugary biomass resource manufacturing. Although numerous scientists worldwide have made varying contributions to artificial sugar synthesis, achieving highly efficient and precise artificial sugar production remains challenging.
Breakthrough in Artificial Sugar Synthesis
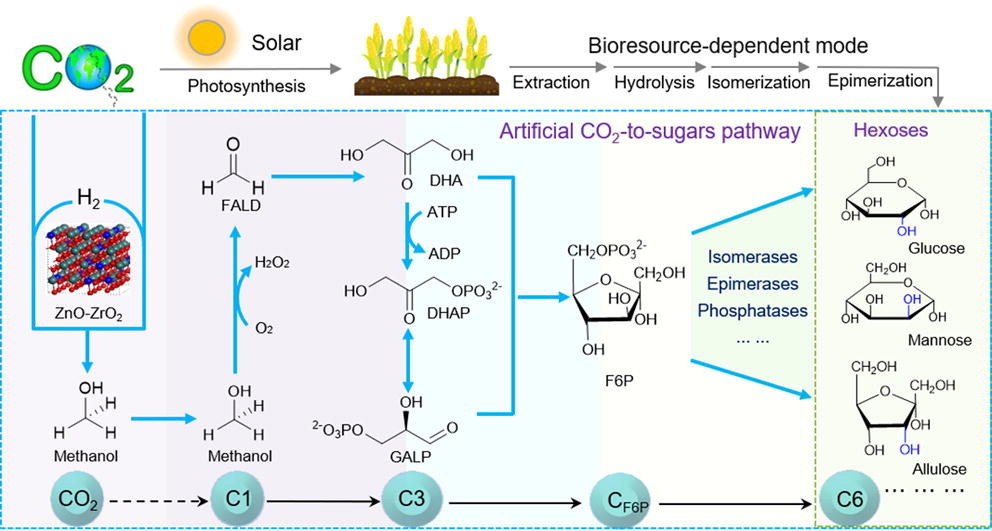
In September 2021, the Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, made a groundbreaking achievement by successfully synthesizing starch from carbon dioxide in the laboratory. Less than two years later, on August 16, the same laboratory realized the precise total synthesis of sugar from carbon dioxide, marking a critical step towards artificial sugar synthesis. This latest research was published in the renowned academic journal, Science Bulletin.
Collaborating with the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, the team established a chemical-enzyme cascading conversion pathway for the artificial synthesis of hexose from carbon dioxide. Using enzyme molecule modification technology, they enhanced the natural enzyme activity and substrate specificity. The team constructed three functional modules - Carbon one, Carbon three, and Carbon six - and achieved precise control over the synthesis of different structures and functions of hexose through modular assembly.
The entire experiment took approximately 17 hours. Compared to traditional methods of extracting sugars from crops like sugarcane, this method leaps from "years" to "hours" in sugar acquisition.
The efficiency of sugar synthesis was 0.67 grams per liter per hour, more than ten times higher than known results, reaching the highest level of artificial sugar production known both domestically and internationally. The carbon fixation synthesis efficiency of glucose reached 59.8 nanomoles of carbon per milligram of catalyst per minute, higher than the chemically synthesized sugar and electrochemical-yeast fermentation coupled method for synthesizing sugar.
This artificial sugar synthesis system can further couple with other enzyme-catalyzed biochemical reactions to prepare structurally diverse sugar molecules and their derivatives. Therefore, this research has established a method for synthesizing hexose from carbon monoxide compounds such as carbon dioxide, methanol, and formaldehyde, achieving a higher conversion efficiency and precisely controllable structure of hexose artificial synthesis. This offers a possibility for transforming non-biomass raw materials into a diverse range of artificial sugar products.
The Future of Artificial Sugar Synthesis
The researchers also achieved precise control over artificial sugar synthesis. "By controlling the different catalytic effects of different enzymes, we can theoretically synthesize almost any type of sugar," said Yang Jiangan, the first author of the paper.
This research provides a pathway that is not dependent on land or cultivation, creating a synthetic route that does not exist in nature. This route is shorter, requires less energy, is more efficient, and can precisely control the structure of the product, improving product selectivity and reducing subsequent separation costs.
Manfred Reetz, a member of the German Academy of Sciences, commented that transforming carbon dioxide into sugar is a particularly challenging task. This achievement provides a flexible, multifunctional, and efficient route for sugar synthesis, opening the door to green chemistry.
Moving forward, the research team aims to further synthesize oligosaccharides, glycosides, or sugar alcohols to obtain sugar molecules that are rare or even non-existent in nature. These molecules could serve as raw materials in fields like food, medicine, and biomanufacturing.
In Chinese, "drinking northwestern wind(喝西北风)” signifies starving poverty. But with such advances, scientists could one day turn even the air into a meal!